
Contributions
Abstract: EP548
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
R-CHOP is the standard first-line therapy of DLBCL. Although over half of patients have been cured under R-CHOP, the remaining patients shared poor prognosis with median OS less than 1 year when progression after front-line treatment. Zanubrutinib, a second-in-class inhibitor of BTK, disrupts BCR signaling and decreases NF-κB activity. Lenalidomide is an oral immunomodulatory agent which downregulates IRF4 and MYD88 signaling. Both two drugs have demonstrated activity in DLBCL.
Aims
To evaluate the safety and efficacy of zanubrutinib, lenalidomide plus R-CHOP (ZR2-CHOP) as the treatment for DLBCL patients, we conducted this single-arm retrospective observational study.
Methods
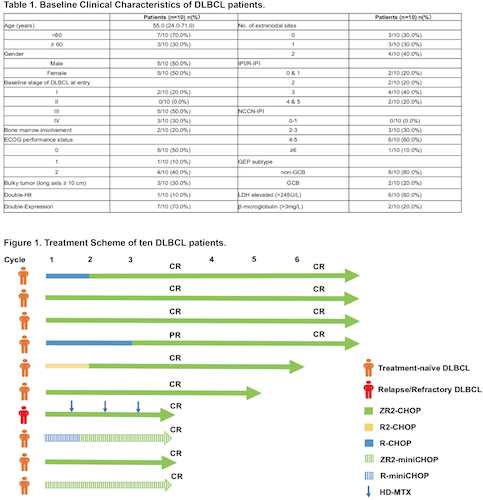
We enrolled patients aged 18 to 75 years old with high-risk DLBCL (including but not limited to double-hit, double-expression DLBCL). Oral zanubrutinib was given continuously (160mg twice daily) from Day 0, lenalidomide 25mg daily Day 1-7. Patients were administered intravenously rituximab (375mg/m2 on Day 0), cyclophosphamide (750mg/m2on Day 1), doxorubicin (50mg/m2 on Day 1), vincristine (1.4mg/m2 on Day 1), and oral prednisone (50mg/day Day 1-5). All patients were recommended to receive 6 cycles of ZR2-CHOP (R-CHOP or R2-CHOP were allowed in cycle 1-2 due to poor physical condition at treatment) and patients older than 70 years old were administered ZR2-miniCHOP (Figure 1). CT or PET-CT scans were conducted to assess mid-term efficacy and assessment after 6 cycles was made by PET-CT scan. ctDNA was dynamically detected before treatment, after 3 cycles and 6 cycles to evaluate tumor mutational burden. The primary endpoint was complete response ratio (CRR) after mid-term and 6 cycles. The secondary endpoint was overall response rate (ORR), ctDNA and the number of adverse events (AE). AEs were graded based on CTCAE (version 5.0).
Results
10 DLBCL patients diagnosed in Pukou CLL Center were enrolled in this cohort between July 2020 and February 2021, with 9 treatment-naïve DLBCL and 1 Relapsed/Refractory DLBCL (PD after 4 cycles of bendamustine plus rituximab). The median age of patients was 55 years old and all patients had ECOG-PS ≤2. 1 patient (1/10) was diagnosed as double-hit DLBCL and 7 patients (7/10) as double-expression. 8 patients were non-GCB and 2 were GCB. 7 patients (7/10) were classified as high-intermediate and high-risk group according to NCCN-IPI (Table 1). At data cutoff (1st March, 2021), the median follow-up was five months (3-8 months) with all patients have completed at least 3 cycles and mid-term assessment could be conducted. The ORR was 100.0%, with 9 patients achieved CR (9/10) and 1 patients achieved PR (1/10). ctDNA was dynamically detected in five patients. The median number of baseline somatic mutation was 5 and all 5 patients showed undetectable ctDNA after 3 cycles. 4 patients have received 6 cycles, all of them achieved CR (4/4) and undetectable ctDNA (4/4) (Figure 1). The most common hematological toxicity events were lymphocytes count decreased, neutrophil count decreased, thrombocytopenia and anemia, with 3-4 level occurrence rate was 70.0%, 30.0%, 20.0% and 20.0%. The most common non-hematological toxicity events were nausea and fatigue. One patients discontinued oral zanubrutinib and lenalidomide more than seven days due to drug rash.
Conclusion
ZR2-CHOP for high-risk DLBCL patients with fair physical condition could achieve high CRR and high proportion of early-stage undetectable ctDNA. The overall tolerability was under control. ZR2-CHOP could be one of the promising choice for the treatment of high-risk DLBCL.
Keyword(s): Diffuse large B cell lymphoma
Abstract: EP548
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
R-CHOP is the standard first-line therapy of DLBCL. Although over half of patients have been cured under R-CHOP, the remaining patients shared poor prognosis with median OS less than 1 year when progression after front-line treatment. Zanubrutinib, a second-in-class inhibitor of BTK, disrupts BCR signaling and decreases NF-κB activity. Lenalidomide is an oral immunomodulatory agent which downregulates IRF4 and MYD88 signaling. Both two drugs have demonstrated activity in DLBCL.
Aims
To evaluate the safety and efficacy of zanubrutinib, lenalidomide plus R-CHOP (ZR2-CHOP) as the treatment for DLBCL patients, we conducted this single-arm retrospective observational study.
Methods
We enrolled patients aged 18 to 75 years old with high-risk DLBCL (including but not limited to double-hit, double-expression DLBCL). Oral zanubrutinib was given continuously (160mg twice daily) from Day 0, lenalidomide 25mg daily Day 1-7. Patients were administered intravenously rituximab (375mg/m2 on Day 0), cyclophosphamide (750mg/m2on Day 1), doxorubicin (50mg/m2 on Day 1), vincristine (1.4mg/m2 on Day 1), and oral prednisone (50mg/day Day 1-5). All patients were recommended to receive 6 cycles of ZR2-CHOP (R-CHOP or R2-CHOP were allowed in cycle 1-2 due to poor physical condition at treatment) and patients older than 70 years old were administered ZR2-miniCHOP (Figure 1). CT or PET-CT scans were conducted to assess mid-term efficacy and assessment after 6 cycles was made by PET-CT scan. ctDNA was dynamically detected before treatment, after 3 cycles and 6 cycles to evaluate tumor mutational burden. The primary endpoint was complete response ratio (CRR) after mid-term and 6 cycles. The secondary endpoint was overall response rate (ORR), ctDNA and the number of adverse events (AE). AEs were graded based on CTCAE (version 5.0).
Results
10 DLBCL patients diagnosed in Pukou CLL Center were enrolled in this cohort between July 2020 and February 2021, with 9 treatment-naïve DLBCL and 1 Relapsed/Refractory DLBCL (PD after 4 cycles of bendamustine plus rituximab). The median age of patients was 55 years old and all patients had ECOG-PS ≤2. 1 patient (1/10) was diagnosed as double-hit DLBCL and 7 patients (7/10) as double-expression. 8 patients were non-GCB and 2 were GCB. 7 patients (7/10) were classified as high-intermediate and high-risk group according to NCCN-IPI (Table 1). At data cutoff (1st March, 2021), the median follow-up was five months (3-8 months) with all patients have completed at least 3 cycles and mid-term assessment could be conducted. The ORR was 100.0%, with 9 patients achieved CR (9/10) and 1 patients achieved PR (1/10). ctDNA was dynamically detected in five patients. The median number of baseline somatic mutation was 5 and all 5 patients showed undetectable ctDNA after 3 cycles. 4 patients have received 6 cycles, all of them achieved CR (4/4) and undetectable ctDNA (4/4) (Figure 1). The most common hematological toxicity events were lymphocytes count decreased, neutrophil count decreased, thrombocytopenia and anemia, with 3-4 level occurrence rate was 70.0%, 30.0%, 20.0% and 20.0%. The most common non-hematological toxicity events were nausea and fatigue. One patients discontinued oral zanubrutinib and lenalidomide more than seven days due to drug rash.

Conclusion
ZR2-CHOP for high-risk DLBCL patients with fair physical condition could achieve high CRR and high proportion of early-stage undetectable ctDNA. The overall tolerability was under control. ZR2-CHOP could be one of the promising choice for the treatment of high-risk DLBCL.
Keyword(s): Diffuse large B cell lymphoma


